Active TB progression estimation
Nathan Green
2018-04-04
This vignette steps through how we estimate the unobserved active TB progression.
Previous studies
There are few references for active TB progression data. We use the observed incidence in Aldridge et al. (2016) (taken directly from the plots) and the tail probabilities from Sutherland, Švandová, and Radhakrishna (1982)
The counts in the Lancet paper are
data("incidence_Lancet", package = "LTBIscreeningproject")
plot(incidence_Lancet$mean, type = 's', ylim = c(0,300), xlab = "Year", ylab = "Incidence")
lines(incidence_Lancet$lower95, type = 's', lty = 2)
lines(incidence_Lancet$upper95, type = 's', lty = 2)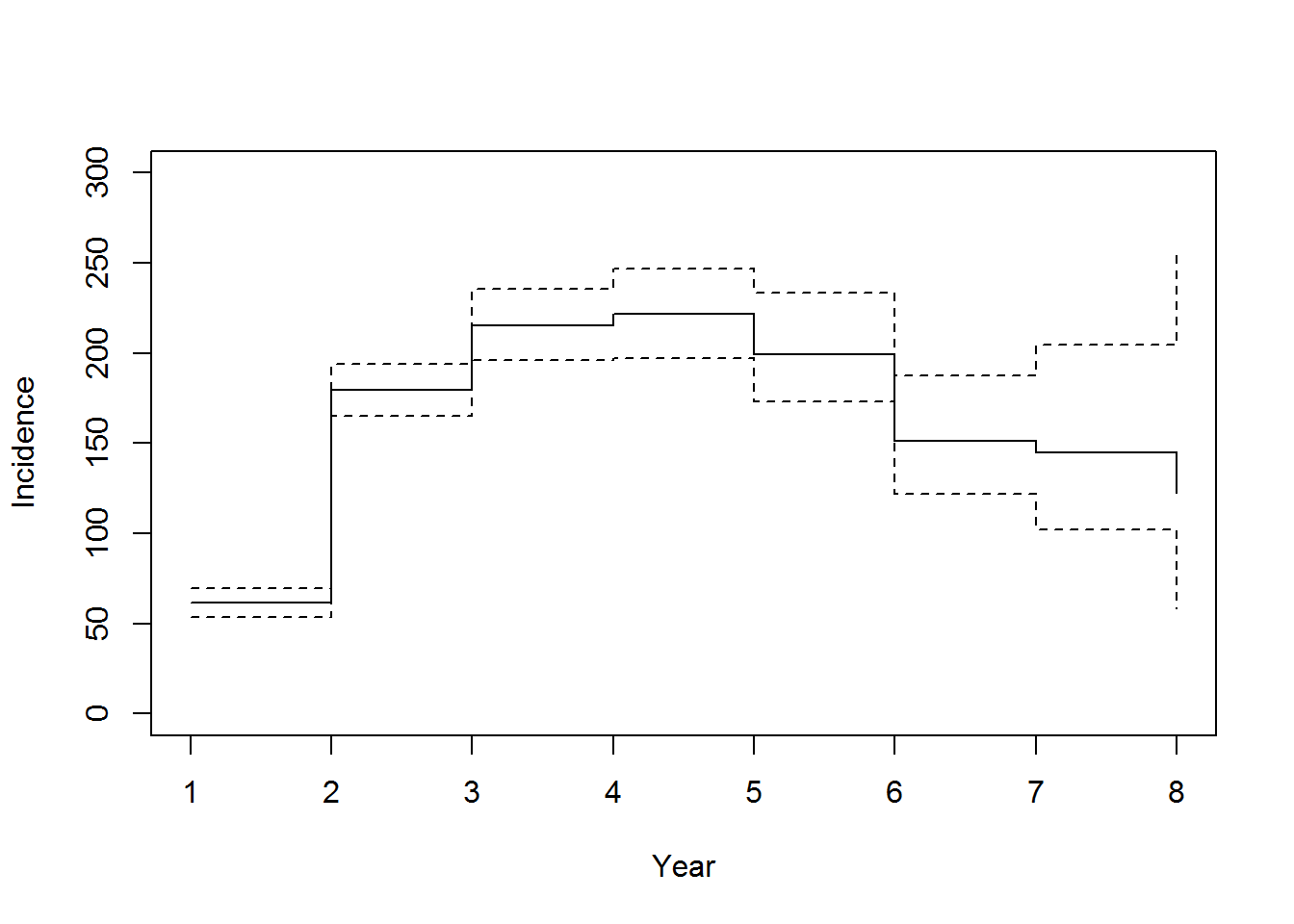
Cases of tuberculosis notified in migrants to EWNI by time since entry. Aldridge et al. (2016)
Extrapolation
We rescale these counts to proportions. We also fit an exponential distibution to the tail of the Lancet data.
All of the data can be put together.

Imputation
We now want to impute individual unobserved progression times to active TB for the cohort. These are times after exiting UK or after end of follow-up.
We scale the progression probabilities using a weight average of LTBI cohort probabilities, because the progression probabilities generate above are for all individuals whereas we know someone’s LTBI status (because we simulate them in the first place). The valued calculated for the cohort is 0.278.
We calculate separately the times for those that leave EWNI and after end of follow-up.
There are a few ways that we could have done this. We could have sampled the progression time and if its after the death time then assume no progression. The problem with this is that we have little say on how many people progress. This is fine if the hazards are independent but the progression probabilities that we’re using are in the presence of competing risk of all-cause mortality. We could just ignore this of course which may be reasonable in some circumstances like when the death rate is low e.g. at younger ages.
An alternative way of generating progression times is by a two-step mixture model-type approach. That is, we first sample whether someone progresses and then we sample the time of progression. In this way we can control the proportion of progressors.
Construct the time to active TB as a mixture model with 2 components.
\[ p(\text{infected}) \times p(\text{infected at $T=t$ | infected}). \] The conditional probability \(p(\text{infected at $T=t$ | infected})\) is produced from Sutherland () and extrapolating assuming an exponential decay to 1 in lifetime. The marginal probability \(p(\text{infected})\) is the chance of being infected in a lifetime.
The reason to do it like this is so that we can easily see the value of \(p(\text{infected})\) for different estimates found in literature, without modifying \(p(\text{infected at $T=t$ | infected})\).
From Pareek et al. (2011), they chose a conservative rate for LTBI to active TB of 5% over 20 years. Marks et al. (2000) calculated a progression rate of 6.7% over 40 years. Choudhury and West (2013) estimated 13% over 10 years or 16.3% over 15 years. 2006 NICE economic appraisal said LTBI testing is cost-effective over 18% chance over 15 years and 2001 NICE economic appraisal reduced this to 12%.
The algorithm is as follows:
- Check that they are LTBI and that the first event is exiting EWNI or end of followup. If not stop.
- Sample \(U \sim Unif[0,1]\).
- If \(U>lifetimeRisk\) then no progression. Stop.
- Otherwise TB progression.
- If followup time is the same as death time return followup time. Stop.
- Sample from ECDF of TB progression times until \(t_{tb} < t_{death}\).
- End.
Here are the original Aldridge et al. (2016) plotted data and an example of our imputation.


Updating cohort
Using the above results the main cohort data set is then updated with this extra information, accounting for the order of events:
- Are they screened before exit EWNI/end of followup/death?
- Time from EWNI entry to TB notification.
- Time from TB notification to death.
- Age (group) at TB notification.
- Are they a TB case-fatality?
- Total QALY to time of death/exit EWNI.
- Discount at time of notification/secondary infection.
This is loaded by
data("model_input_cohort", package = "LTBIscreeningproject")References
Aldridge, Robert W., Dominik Zenner, Peter J. White, Elizabeth J. Williamson, Morris C. Muzyamba, Poonam Dhavan, Davide Mosca, et al. 2016. “Tuberculosis in migrants moving from high-incidence to low-incidence countries: a population-based cohort study of 519 955 migrants screened before entry to England, Wales, and Northern Ireland.” The Lancet 388 (10059). The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY license: 2510–8. doi:10.1016/S0140-6736(16)31008-X.
Choudhury, I W, and C R West. 2013. “The outcome of a cohort of tuberculin-positive predominantly South Asian new entrants aged 16 – 34 to the UK : Blackburn 1989 – 2001” 36 (3): 390–95. doi:10.1093/pubmed/fdt110.
Marks, Suzanne M, Zachary Taylor, Noreen L Qualls, Robin J Shrestha-kuwahara, Maureen A Wilce, and Cristy H Nguyen. 2000. “Outcomes of Contact Investigations of Infectious Tuberculosis Patients” 162: 2033–8.
Pareek, Manish, I. Abubakar, Peter J. White, G. P. Garnette, and A. Lalvani. 2011. “Tuberculosis screening of migrants to low-burden nations: Insights from evaluation of UK practice.” European Respiratory Journal 37 (5): 1175–82. doi:10.1183/09031936.00105810.
Sutherland, Ian, Eva Švandová, and S. Radhakrishna. 1982. “The development of clinical tuberculosis following infection with tubercle bacilli.” Tubercle 63 (4): 255–68. doi:10.1016/S0041-3879(82)80013-5.